(Heads Up! I’m Sneaking in a YAN and Aroma/Flavor Discussion Here)
This post is a part of the ongoing “Back to Enology” series in which I, Denise Gardner, take time to explain the science behind concepts misunderstood (or mythological) in winemaking practices.
- Part 1 discussed Misconceptions around sulfur dioxide additions to wine.
- Part 2 talks about the sophistication with developing RTD Cocktails/Formula wines so that they taste good and are scalable.
A full reasoning behind this “Back to Enology” series can be found at the end of today’s blog post.

Photo by: Denise M. Gardner
With harvest season on the horizon for many, I thought I would take this month to talk more in depth about the development of hydrogen sulfide (H2S), and the common way of remediating it: the addition of copper sulfate. As a wine consultant, I find that there are a lot of schools of thought surrounding this topic among winemakers. For many, avoiding the development of hydrogen sulfide is just “too hard” or “too complicated” or “too costly.” Don’t worry, I’ll debunk that later. For others, there’s a lack of understanding why we want to avoid the addition of copper sulfate, in the name of wine quality, especially today with the rise of screw cap or can packaging, thiol-producing yeast strains, and consumer perceptions on heavy metals in wine.
Are you ready to dive into this topic a bit more with me?
(Author’s Note: This post contains a lot of chemical names. Don’t fret. There is not a test at the end.)
What are Hydrogen Sulfide and Thiols/Mercaptans
In order to understand the need for copper sulfate as a fining agent, we must first talk about what hydrogen sulfide and thiols/mercaptans are so that we understand what copper sulfate reacts with in wine.
Thiols and mercaptans (they are the same class of compounds, different names depending on what year you are looking at literature) are volatile (odorous) compounds that contain a -SH component in their chemical structure. The “-SH” stands for “sulfur-hydrogen”. Some common thiol compounds in wine include methanthiol (methiol) and ethanethiol (ethyl mercaptan). The generation of these compounds is believed to occur during primary fermentation (Swiegers et al. 2005) though there is still a lot of research pertaining to the generation of these compounds in wine.
Additional sulfur-based volatile compounds can contribute to wine aroma and flavor, including sulfur dioxide (SO2), thioesters, sulfides (or thioethers, and this group includes disulfides), and heterocycles (which often develop from compounds like thiamin) (Etievant 1991). Some common wine sulfides (thioethers) include dimethyl sulfide (DMS, (methylsulfanyl)methane)) and diethyl sulfide (DES, (ethylsulfanyl)ethane) while di- and tri-sulfides include dimethyl disulfide (DMDS, ((methyldisulfanyl)methane) and dimethyl trisulfide (DMTS, dimethyltrisulfane) (Bisson, 2009). The only thing all of these different classes of volatile compounds have in common is that the element “sulfur” is a part of their chemical structure. Otherwise, thiols/mercaptans are chemically structured quite differently from disulfides and so forth.
Sulfur-based aromatic compounds have several attributes in common. The first is that many of them are highly reactive. This makes it incredibly difficult to study sulfur-based aromatic compounds because they easily react and chemically change with a lot of things. The chemical reactions they undergo can temporarily or permanently inactivate their associated aroma. The second attribute sulfur-based aromatics have in common is that our individual ability to sense these various compounds varies significantly. I still remember being humbled by the fact I found a Syrah overly delightful, smelling and tasting like raspberry jam while the remainder of colleagues at my table all smelled “canned corn” from the same exact wine. If you want to embark in a humbling experience, learning how you can and cannot smell various aroma compounds at various concentrations is a fantastic exercise!
My point of telling this is to remind you that just because you cannot smell or taste something does not mean another person cannot smell or taste something. Your “good wine” could be someone else’s “bad wine”.
The Good and the Bad of Thiols
In general, the “rule of the land” through the mid-1990s had been that sulfur-based volatile compounds, listed above, do not contribute positive aromas or flavors to wine. Thiols were generally considered “negative” wine aromas.
Then in the early 2000’s, the generation of thiol-rich wine varietals had emerged. The easiest example that illustrated this point was New Zealand Sauvignon Blanc. The New Zealand Sauvignon Blanc style emphasized the thiol-based varietal character of the Sauvignon Blanc grape. By retaining the sulfur-based thiol aromatics through fermentation and processing, the wines became rich in aromas and flavors with tropical fruit like mango or starfruit, gooseberry, freshly cut green grass, and grapefruit essences. What the New Zealand industry had discovered and commercialized was the production of a thiol-rich variety and displaying the aromatic thiols through specific processing operations and packaging selections that retained the aromatic thiols.
Since New Zealand Sauvignon Blanc’s popularity, other wine grape varieties have been found to contain aromatic thiols as part of their varietal character. This included the discovery of thiol aromatics in Provençal rose wines, which we will circle back to later.
It’s important to note that for any aromatic compound, their existence in a substance does not equate to their perception. For most aromatic compounds, a detection threshold concentration has to be reached in order to be perceived by the human nose. Furthermore, in order to identify the compound as the accurate compound/scent, a recognition threshold (often greater than the detection threshold) must be reached. If that isn’t complicated enough, the expression of a compound also depends on the matrix (the substance the compound is a part of) and synergism associated with other aromatic compounds. Sometimes, the presence of two different aromatic compounds will not express the aromas associated with the individual parts. Instead, a third unique aroma arises. Fascinating and yet complicated, isn’t it?
The aromatic thiols associated with Sauvignon Blanc that became most heavily discussed in research included 4MMP (4-mercapto-4-methylpentan-2-one or 4-methyl-4-sulfanylpentan-2-one), 4-MMPOH (4-mercapto-4-methylpentanol), 3MH (3-mercaptohexan-1-ol), and 3MHA (3-mercaptohexyl acetate). 4-MMPOH is an alcohol derivative of 4MMP while 3MHA is an ester of 3MH (Bisson, 2010). 4-MMP and 4-MMPOH are responsible for aromas like “box tree” at concentrations less than 70 ng/L (Roland et al. 2011), “cat urine”, and “broom shrub plants” while 3MH and 3MHA are responsible for aromas of “passion fruit”, “gooseberry”, “grapefruit”, and “guava” (Bisson, 2010; Roland et al. 2011). The initial thiols, 4MMP and 3MH, are present in the grape berry skins (Bisson, 2010) and released during primary fermentation.
Since the discovery of volatile thiols, several wine grape varieties have been noted to contain them. The most ubiquitous (based on where they arrive from during fermentation) are 3MH and 3MHA. Varieties, beyond Sauvignon Blanc, and wines found to contain 3MH and 3MA, include Provençal rosès (Masson and Schneider, 2009), Petit Manseng, Gros Manseng, Semillon, Verdejo, Grenache, Merlot, and Cabernet Sauvignon (Roland et al. 2011). 4MMP was also found in Maccabeo, Gewürztraminer, Riesling, Muscat, and Petit Manseng (Roland et al. 2011). While there are additional varieties and wines identified with these varietal thiols, I’ve tried to focus on those that are most familiar to everyone here.
Where do Hydrogen Sulfide and Thiols come from?
I think it’s important to know where hydrogen sulfide (H2S) and thiols/mercaptans come from in wine because there has been a lot of discoveries in the research literature over the years that actively discuss sulfur-based aroma compounds. Some of this is due to interest and the other part is due to changes in technology that provide better research tools for measuring or documenting sulfur-based aromatic compounds.
From what I understand, today the general school of thought is that the generation of hydrogen sulfide is primarily through the sulfate reduction pathway, shown below, in yeasts during primary fermentation. The sulfate reduction pathway is a way for yeast to build or breakdown sulfur-containing amino acids, which are required for yeast cell protein development or degradation. Under amino acid building principles, sulfate (SO42-) is actively brought into the yeast cell via the surrounding juice, undergoing a series of chemical reactions until it is made into sulfide (S2-). Sulfide is then used in combination with homocysteine, which makes both of the amino acids, cysteine and methionine. For cysteine and methionine production to continue within the yeast cell, an equal amount of nitrogen (from the nitrogen compound pool within the yeast cell) has to be available. This is essential because the building blocks of amino acids are nitrogen. If there is not enough nitrogen available to continue building amino acids, then the sulfide starts concentrating within the yeast cell. Sulfide is actually toxic to the yeast. Thus, if sulfide starts to accumulate within the yeast cell, then the yeast cell will diffuse it over its membrane and into the fermenting wine.
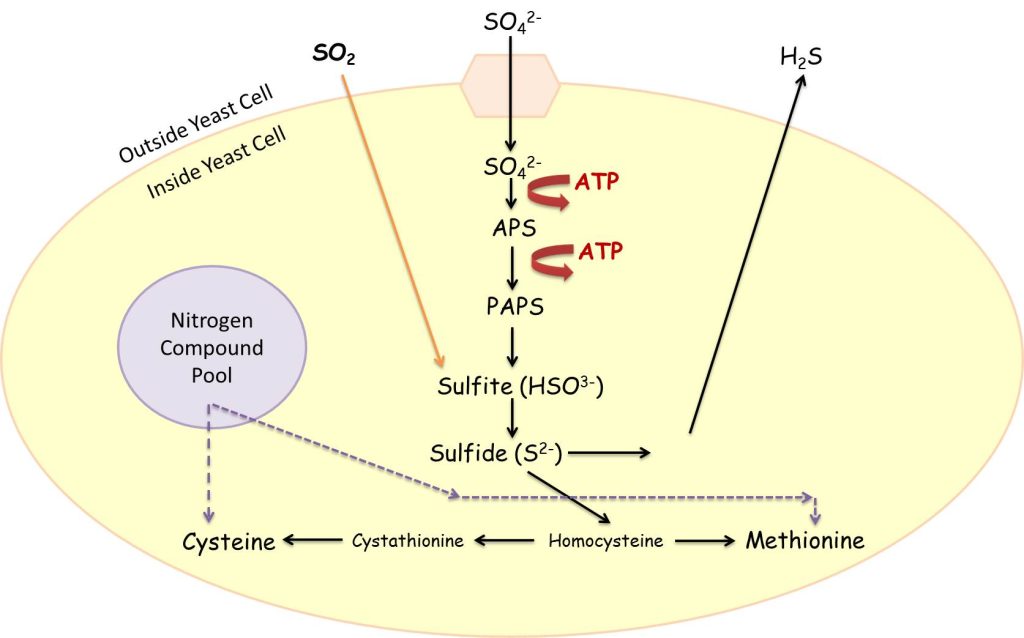
The other thing to note about the sulfate reduction pathway is that sulfur dioxide (SO2) bypasses a whole bunch of energy-requiring steps in the sulfate reduction pathway and gets easily transformed into sulfite, the precursor to sulfide. This means that high levels of sulfur dioxide in the fermenting wine can get utilized by yeast cells and accumulate higher concentrations of sulfide development. Obviously this can be a sticky situation as too much sulfide development by yeast will end up in the wine. In fact, studies have shown that when larger additions (>80 ppm SO2) of potassium metabisulfite are added to a grape must, hydrogen sulfide concentrations in the wine increase.
We see this in various stages through sensory detection during fermentation. Hydrogen sulfide is often detected (Bisson, 2009):
- Early during primary fermentation (within about 2 – 4 days), which is likely caused by an early-onset nitrogen imbalance.
- Towards the very end of fermentation, which is likely caused by the degradation of sulfur-containing amino acids or a stress response due to an ethanol intolerance.
For wines that undergo sur lie aging, hydrogen sulfide can also develop during this stage. Here, hydrogen sulfide detection is often due to yeast autolysis (Bisson, 2009) in which sulfur-containing compounds are released by the yeast cells.
But what about some of these more complicated, varietal thiols? As discussed previously, 4MMP and 3MH are a part of the flavor components bound in grape skins (Roland et al. 2011). (Side note: aroma and flavor compounds within a grape are source from the grape’s skin, in general.) The generation and accumulation of these aromatic thiols comes from the yeast via lyase enzymes, accounting for 1 – 3% of most Saccharomyces yeast strain enzymatic activity (Bisson, 2010; Roland et al. 2011).
Other sulfides and disulfides may generate from sulfur-containing amino acids, sulfur-containing vitamins, and glutathione (contains cysteine, which contains sulfur) (Bisson, 2009). Research continues to develop in this area to discover a firmer understanding of how this happens in wine (Waterhouse, Sacks, and Jeffery, 2016).
What does all of this mean for the winemaker? Let’s make it practical!
The first point to this extra long rant to is to declare winemakers actually have some control over thiol and hydrogen sulfide development.
Let’s start with hydrogen sulfide, which is regulated by nitrogen availability during primary fermentation. If winemakers know the starting must/juice nitrogen content and the nitrogen needs for a particular yeast strain, then, guess what? They can manage nitrogen additions during primary fermentation to reduce the incidence rate of hydrogen sulfide development.
Thus, yeast available/assimilable nitrogen (YAN) is fairly important and a point of winemaker control. (Don’t worry, I’m going to come full circle here and show another reason why YAN is important with copper sulfate additions, too.)

Photo by: Denise M. Gardner
Every supplier provides nutrient recommendations for their nutrients and yeast strains today. This is a testament to how effective nutrient additions are in reducing hydrogen sulfide during primary fermentation. And, look. There will be times when hydrogen sulfide development happens. High YAN fermentations tend to be a big problem, for example. I’m also anecdotally noticing different nutrient requirements for hybrid wine grape fermentations vs. Vitis vinifera fermentations. In other words: there’s still more work to do and more conclusions to uncover in this area of science. But the point is that treating fermentations for their nutrient needs is a way to enhance wine quality that all wineries are able to do.
I know most small wineries will make the argument that it is too expensive to run YANs. It is certainly true that for many wineries east of California, getting YANs is harder and probably more expensive than for those wineries in the Western U.S. But… it’s still feasible to get YANs in a reasonable, cost-effective time frame. And luckily, I wrote an article on this several years ago explaining to all who read it just how to get YANs when it’s a little more challenging for you: Fermentation Nutrition: What to Know and Why to Know It. You can do it! I believe in you! With very little planning, you can easily set up a system during harvest season to get YANs because most of the time, nutrient alterations are not absolutely necessary until the 1/3-sugar depletion time point through primary fermentation.
I know it’s not inexpensive (ETS is currently running at $60-$66/sample depending on the number of samples submitted). However, a 1-L container of 10% food-grade copper sulfate is just under $30 + the time it will take to do bench trials and add the copper sulfate to the wine. Plus, the cost you probably spent on yeast (about $50-$60/500-g of yeast). And the whole point of that yeast strain, beyond getting through fermentation, is to generate all those fancy flavor descriptors so many winemakers pay attention to in the supplier winemaking handbooks. Yeast nutrients are about $100+/1-kg bag. Plus add in all the time you spent taking care and monitoring the fermentation. Without knowing YAN, and the requirements by the yeast strain you selected, you’re essentially starting to throw $1 bills into that fermentation tank and hoping it pays off. Sometimes, it will pay off. Sometimes, it won’t.
With those dollar signs running around in our heads, I want to bring attention to one more point. With the popularity of thiol-rich wines like New Zealand Sauvignon Blanc (and many other varieties that are now processed in a New Zealand way), came the development and commercialization of thiol-producing yeast strains. Many of these yeast strains are marketed to enhance the varietal thiol aromas and flavors (3MH and 4MMP) beloved in New Zealand Sauvignon Blanc. But, these yeast strains also typically come with a hefty price tag. Alchemy, for example, is about $120/1-kg.
Furthermore, the development of these aromatic thiols are reliant on proper nutrient management. Without measuring YAN, the winemaker is playing with flavor-fire, right? Because if the fermentation develops hydrogen sulfide, how do we fix it? Copper sulfate additions. (And this is the second reason as to why YAN is so important to measure and manage.)
What aromatic compounds does copper sulfate react with and inactivate their aromatics? Hydrogen sulfide and aromatic thiols.
Thus, if you are fermenting a thiol-rich variety, and if you are using a high thiol-producing yeast strain, and the fermentation results with hydrogen sulfide, not only will you have to eliminate the hydrogen sulfide aroma with copper sulfate, but the copper sulfate will also reduce a lot of that varietal aroma that you just invested so much into. Hence, there is a net negative effect on the wine under these conditions as the wine will lose much of that beloved aroma and flavor associated with sulfur-containing compounds.
So then… should we use copper sulfate?
When I talked about this topic publicly, I actually started by discussing how so many winemakers don’t think it’s a big deal to use copper sulfate. And really, it isn’t. If you need it, use it.
But my point is: don’t make yourself use copper sulfate if you don’t have to. There are tools, today, that can more easily prevent hydrogen sulfide formation in wines that winemakers did not have access to prior to the year 2000. There’s no point, in my opinion, in erasing progress. All winemakers have access to the tools: use them.
Our previous understanding with copper sulfate was that its additions to a wine bound to a thiol or hydrogen sulfide and then the bounded-hydrogen sulfide or bounded-thiol precipitated (fell) out of the wine in the lees. Rack the wine and no more problems would arise. But recent research by Kreitman et al. 2016A and Kreitman et al. 2016B has shown that copper remains in a soluble state within the wine, even when bound to thiols or hydrogen sulfide. This means the copper doesn’t necessarily fall out of the wine like we all once thought.
Avoiding copper sulfate additions, therefore, tends to be the way to go. The reasoning for this is because if the copper remains in the wine, two things can occur. One, the copper concentration of the wine is increased (though the U.S. does regulate maximum allowable copper concentrations in wine). And, two, copper, like other heavy metals, can catalyze oxidation-reduction reactions in wine under ideal circumstances.
Now, what are those ideal circumstances?
Well, that’s a lot of oxidation-reduction chemistry I don’t think you want to read from me today. I’m not the right person to explain that. But I can say this, under many forms of screw capped bottles, sulfur-compounds are more easily released, which create a reduced-aromatic odor (Bisson, 2009; Waterhouse, Sacks, and Jeffrey, 2016). Roland et al. 2011 noted significant aroma losses noted in studies that reviewed thiol-rich varieties bottled under cork closures. Even under screw caps, Herbst-Johnstone, Nicolau, and Kilmartin (2011) found a 29-46% loss of varietal thiol concentrations within 3 months post-bottling. That’s a huge amount of loss when we are talking about aromatics.
I fully support the use of screw caps with wine bottles. Love the trend. But I also very regularly consult with my clients about reducing copper concentrations if copper sulfate was used to treat the wine with hydrogen sulfide. I do this even if the copper concentrations are slightly below the current maximum allowable copper concentration in finished wine. Why do I do this?
Because anecdotally, again, I’ve noticed an incredibly high incidence of hydrogen sulfide from:
- Screw capped wines,
- Rosès, and
- Whites, especially aromatic whites.
(By the way, I highly recommend checking residual copper concentration prior to packaging, which is $34/test, and contributes to the overall cost of dealing with this issue.)

Photo by: Denise M. Gardner
My gut tells me the higher incidence rate of reduced-thiols in the aroma of wines is partially due to a number of factors:
- A heavy winemaking focus on boosting (varietal) thiols, especially in the wine yeast realm. Remember, there are “good” and there are “bad” thiols, and reactions can occur quickly taking things from good-to-bad within minutes. (You don’t have to believe me, here. You can prove it to yourself. Leave a bottle of New Zealand Sauvignon Blanc opened for an hour. Does it taste as fruity as it did when you first opened it? Or do you now sense a stink?)
- A lack of winemakers being able to reliably sense hydrogen sulfide/reduced thiols, or thinking consumers won’t sense it. (We sense it. And I buy wine a lot. I don’t usually re-buy reduced wine.)
- Not understanding the chemistry dynamics of wine bottled under screw cap closures vs. cork closures. Believe it or not, packaging decisions have a great affect on wine quality post-bottling. Packaging is certainly an area where we could talk a lot more science in relation to winemaking.
It’s not guess work, it’s science. Even the smallest wineries can use the science to their advantage here. Measure YAN. Make good nutrient additions for each fermentation. Avoid copper sulfate if you can (by avoiding reduced thiol aromatics). Don’t fall for the aeration “solution”… I didn’t get into that here, but it’s not going to fix your thiol problems. And work with someone to help you if you need it because this is heavy science and it may take changing some operations or practices to help reduce the incidence of this issue for you.
Key Take-Aways & Practical Applications
- Measure YAN and treat fermentations specific to the YAN, starting Brix, and yeast strain selection. Use YAN, Part 2 and YAN, Part 3 Winemaking Lessons to discover more detail on these topics.
- Budget in ways to measure YAN for each fermentation. Refer to the free YAN Primer for ways to do this if you are knew to the YAN-measuring scene.
- When using a high-thiol producing yeast strain or fermenting a thiol-rich variety, recognize that copper sulfate additions will also impact the aroma and flavor of the wine negatively if you need to use it post-fermentation.
- Residual copper in a wine is regulated through the TTB. Winemakers can reduce copper concentrations before bottling.
- Residual copper concentrations in wine can lead to the onset of post-bottling oxidation-reduction reactions that can have an impact on wine quality.
- Wines packaged under screw cap closures or in cans need special considerations with regards to copper concentrations.
Back to Enology…
To quote physicist Lisa Randall, “You can’t have these executive committees or congressional committees that really understand things. I mean, some things are difficult to understand, and not everyone will understand them. And it’s really important for a scientist to be able to at least get the information out there and have that taken into account. There’s also an idea that, you know, when people talk about science, they’re being elitist. That’s not what it’s about. It’s about understanding the world. It’s something that we want to share. I mean, there’s a wonderful universe out there.”
I’ve recently been listening to the Freakonomics series on Richard Feynman, of the greatest scientists of modern-day era. In the last of this three-part series, The Vanishing Mr. Feynman, there was a large emphasis on the decline of trusting scientists or scientific information.
I have to admit that I gravitated to this podcast series as I, like many other scientists in many scientific fields, have recently found the dissemination of scientific information a rather challenging uphill battle.
In the lowest of times, I have considered completely giving up on communicating enology information, the scientific understanding of winemaking. But in the highest of times, I recognize that I have to continue to communicate this information for those that simply do not know the science that strive to learn the science or that strive to make better wines using well-documented winemaking principles.
Therefore, over the next few months, I’d like to address a series of – what I call – “winemaking myths and misconceptions” based on commonly communicated ideas/opinions by industry members that are often veiled as scientific fact or industry standard.
Instead, I’ll work on coming back to what we know scientifically about each of these topics.
Fair warning: the science is usually more complicated, less definitive, and sometimes not yet determined compared to the common discussions you may hear in the winemaking community.
With that in mind, I think it’s important to remember that very fact. The science is complicated. The science may not be definitive as scientists continue to research and test hypotheses (questions) related to winemaking, and as technologies improve giving scientists access to possibility of researching more hypotheses. And sometimes, the science is not yet conclusive. Sometimes scientists do not know the answers we may be looking for. And that’s okay. Even in the face of “not knowing all,” I have managed to advise many of my clients into making a fair share of good quality wines, or award-winning wines. Of course, there have also been growing and learning opportunities during those times in which we don’t know the answers.
Ultimately, it is the continuous stream of new information associated with enology that led me to enology in the first place. Winemaking, after all, is a continuous educational experience.
Would you like to learn with me?
Resources that Supported this Blog Post:
Bisson, L. 2009. “The Sulfur Taints.” From, Wine Flavor 101A: Identifying and Reducing Flavor Negatives – Part 1. Live Instruction, December 2009 at UC Davis Campus.
Bisson, L. 2010. “Sulfur-Containing Grape Volatiles.” From, Wine Flavor 101D: The Varietal Contributors. Live Instruction, March 2010 at UC Davis Campus.
Herbst-Johnstone, M., L. Nicolau, and P.A. Kilmartin. 2011. Stability of Varietal Thiols in Commercial Sauvignon Blanc Wines. Am J Enol Vit. 62(4): 495-502.
Kreitman, G.Y., J.C. Danilewicz, D.W. Jeffery, & R.J. Elias. 2016A. Reaction Mechanisms of Metals with Hydrogen Sulfide and Thiols in Model Wine. Part 1: Copper-Catalyzed Oxidation. J. Agric. Food Chem. 64:4095 – 4104.
Kreitman, G.Y., J.C. Danilewicz, D.W. Jeffery, & R.J. Elias. 2016B. Reaction Mechanisms of Metals with Hydrogen Sulfide and Thiols in Model Wine. Part 2: Iron- and Copper-Catalyzed Oxidation. J. Agric. Food Chem. 64:4105 – 4113.
Masson, G. and R. Schneider. 2009. Key Compounds of Provence Rosè Wine Flavor. Am J Enol Vit. 60(1):116-122.
Roland, A., R. Schneider, A. Razungles, and F. Cavelier. 2011. Varietal Thiols in Wine: Discovery, Analysis and Applications. Chem. Rev. 111:7355-7376.
Swiegers, J.H., E.J. Bartowsky, P.A. Henschke, and I.S. Pretorius. 2005. Yeast and Bacterial Modulation of Wine Aroma and Flavor. Aust. J. Grape Wine Res. 11:139-173.
Waterhouse, A.L., G.L. Sacks, and D.W. Jeffrey. 2016. Understanding Wine Chemistry. ISBN: 978-1-118-62780-8.
The views and opinions expressed through dgwinemaking.com are intended for general informational purposes only. Denise Gardner Winemaking does not assume any responsibility or liability for those winery, cidery, or alcohol-producing operations that choose to use any of the information seen here or within dgwinemaking.com.
