
Photo by: Savannah Smith Photography
To quote physicist Lisa Randall, “You can’t have these executive committees or congressional committees that really understand things. I mean, some things are difficult to understand, and not everyone will understand them. And it’s really important for a scientist to be able to at least get the information out there and have that taken into account. There’s also an idea that, you know, when people talk about science, they’re being elitist. That’s not what it’s about. It’s about understanding the world. It’s something that we want to share. I mean, there’s a wonderful universe out there.”
I’ve recently been listening to the Freakonomics series on Richard Feynman, of the greatest scientists of modern-day era. In the last of this three-part series, The Vanishing Mr. Feynman, there was a large emphasis on the decline of trusting scientists or scientific information.
I have to admit that I gravitated to this podcast series as I, like many other scientists in many scientific fields, have recently found the dissemination of scientific information a rather challenging uphill battle.
In the lowest of times, I have considered completely giving up on communicating enology information, the scientific understanding of winemaking. But in the highest of times, I recognize that I have to continue to communicate this information for those that simply do not know the science that strive to learn the science or that strive to make better wines using well-documented winemaking principles.
Therefore, over the next few months, I’d like to address a series of – what I call – “winemaking myths and misconceptions” based on commonly communicated ideas/opinions by industry members that are often veiled as scientific fact or industry standard.
Instead, I’ll work on coming back to what we know scientifically about each of these topics.
Fair warning: the science is usually more complicated, less definitive, and sometimes not yet determined compared to the common discussions you may hear in the winemaking community.
With that in mind, I think it’s important to remember that very fact.
The science is complicated.
The science may not be definitive as scientists continue to research and test hypotheses (questions) related to winemaking, and as technologies improve giving scientists access to possibility of researching more hypotheses.
And sometimes, the science is not yet conclusive.
Sometimes scientists do not know the answers we may be looking for. And that’s okay. Even in the face of “not knowing all,” I have managed to advise many of my clients into making a fair share of good quality wines, or award-winning wines. Of course, there have also been growing and learning opportunities during those times in which we don’t know the answers.
Ultimately, it is the continuous stream of new information associated with enology that led me to enology in the first place. Winemaking, after all, is a continuous educational experience.
Would you like to learn with me?
Misconception: Sulfur Dioxide Equilibrates in Tank (i.e., No Mixing Needed After Adding SO2)
The definition of “equilibrium” is “a state of which opposing forces or influences are balanced.”
In terms of chemistry, an equilibrium would exist within a liquid when the chemical components are balanced, or equal, throughout the volume of the liquid.
Let’s think about this in terms of people instead of chemicals.
Let’s say you go to a movie theater, and two different rooms are showing the same exact movie at the same exact time. Each room holds 100 people. However, one of the rooms is almost packed full of people, and currently 80 people are seated in the theater. The second room contains only 10 seated people in it. Which room will you end up going to sit in?
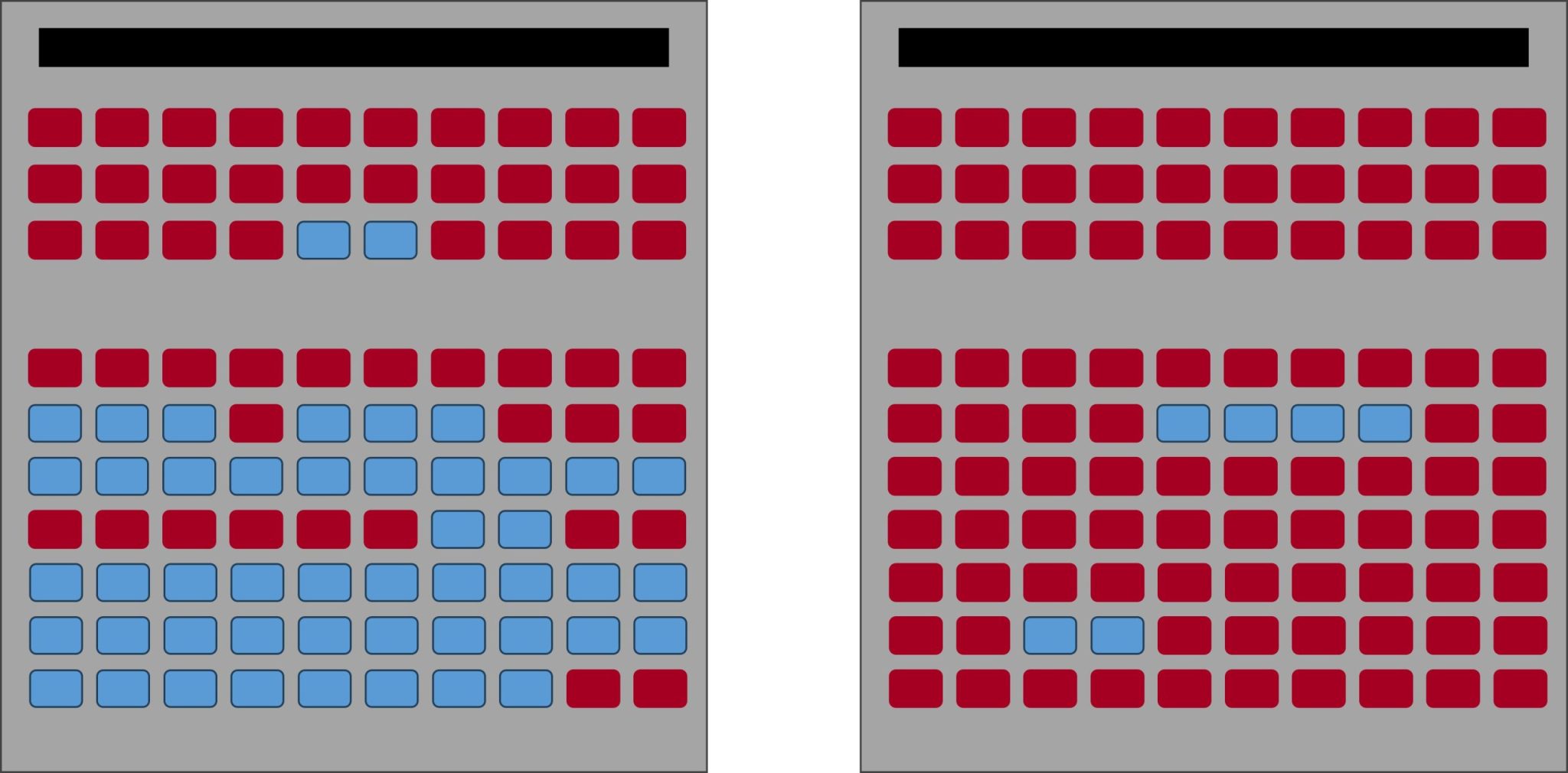
Image by: Denise M. Gardner
I’m sure you’re going to that second room that only contains 10 people. We instinctively gravitate towards the less crowded room.
But let’s say by the time the movie starts, both rooms contain 84 people. During the time in which people were choosing their seats, an additional 74 people ended up in the less-full room while only 4 people found seats in the initial room. Now the rooms are in equilibrium: there are the same number of individuals in both rooms showing the same exact movie at the same exact time.
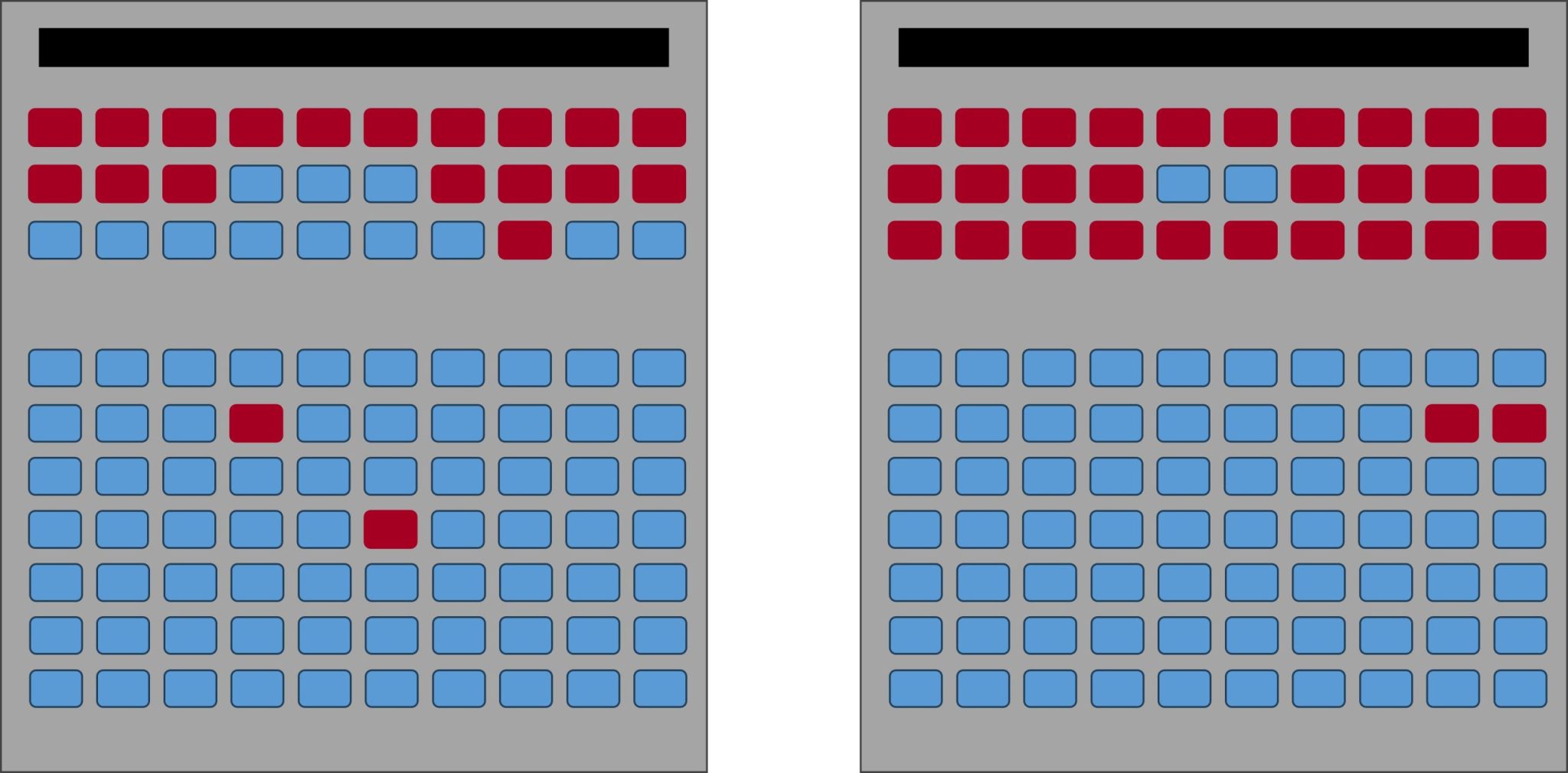
Image by: Denise M. Gardner
Chemicals within liquid mixtures (which are also referred to as, “solutions”) act much the same way. The chemical components will equally spread throughout a mixture. Thus, if potassium metabisulfite is added to a tank filled with wine, in theory, the potassium metabisulfite should split into its subsequent parts (potassium and sulfur dioxide) and equally spread throughout the wine.
However, stratification is also true in wine production. “Stratification” is defined as “the arrangement or classification of something into different groups.”
When a sulfur dioxide addition is made, while the theory of a sulfur dioxide equilibrium should exist, as tank sizes increase, stratification of sulfur dioxide tends to find favor.
Can you see this in production?
Yes.
Many DGW clients have heard me say, “Do me a favor. Measure the free and total SO2 at both the top of your tank and from the racking valve.” The reason I advise this is because I want to eliminate the possibility of SO2 stratification in a tank.
Now, how can you avoid stratification as a winemaker?
Always, always, always, mix in the sulfur dioxide when you are making an addition.
For tanks, the best way to do this is through use of a tank mixer. Many winemakers will result to “fake pump-overs,” which is the process of drawing wine out from the bottom of the tank and pumping back over and beneath the top surface of the tank. While this is an effective way of mixing the wine, fake pump-overs incorporate much more oxygen into the wine than use of a tank mixer.
Also, make sure you are adding sulfur dioxide properly to a wine. The most efficient way of adding sulfur dioxide us by creating a stock solution of sulfur dioxide and then distributing to a wine. However, many people tend measure out potassium metabisulfite and directly add to a wine tank after diluting the potassium metabisulfite in water. If you are doing this, first, stop doing this. Second, if you have to add the potassium metabisulfite directly to the tank, mix in a sample of the wine first. This reduces a water addition and is a more effective way of getting sulfur dioxide into the wine.

Photo by: Savannah Smith Photography
Misconception: A 25 Part SO2 Addition Adds 25 PPM of Free SO2 to the Wine
This general misconception is based upon a lack of understanding how sulfur dioxide breaks into different components in the wine. (Author’s tip: For DGW Clients and Members, if you find yourself confused through this blog section, check out the Winemaking Lesson, “Demystifying Sulfur Dioxide.” The secret is there!)
I will be the first to admit that I cannot explain sulfur dioxide chemistry as well as a wine chemist. However, I’ve also worked really, really hard at trying to capture both a firm understanding of sulfur dioxide chemistry and practical observance in the field/cellar.
One thing I can admit is that the misconception stated above, “a 25 part SO2 addition adds 25 ppm of free SO2 to the wine” is, occasionally, true.
But, 95% of the time, it is not true.
What 5% of the time is it true? When the wine’s free SO2 concentration has already reached the 0.85 ppm (molecular) concentration.
During the other 95% of the times, however, when a 25 ppm (part) SO2 addition is added to the wine, less than 25 ppm of SO2 will go towards the free SO2 concentration.
In a previous Winemakers Research Exchange (WRE) report, Harmon (2019) cited a few winemaking resources that claimed “30– 70% of an SO2 addition” will end up in the free form. The WRE is a non-profit organization in Virginia that runs industry-trials to test various winemaking techniques. It’s important to note that the WRE is not an official scientific body. However, they are running industry trials, which – like any scientific study – has both pros and cons to the study’s design and conclusions. The power in these trials, in my opinion, is that they try to control as many variables as possible in a practical industry setting while testing scientific principles. Again, there are limitations to this approach (e.g., running a sound number of replicates, and thus many of their studies lack statistical rigor), but also great observations (e.g., they are showing actual data from commercial lots of wine in a thought out, planned way).
And, to be fair, their observations about SO2 concentrations are not without harder scientific backing.
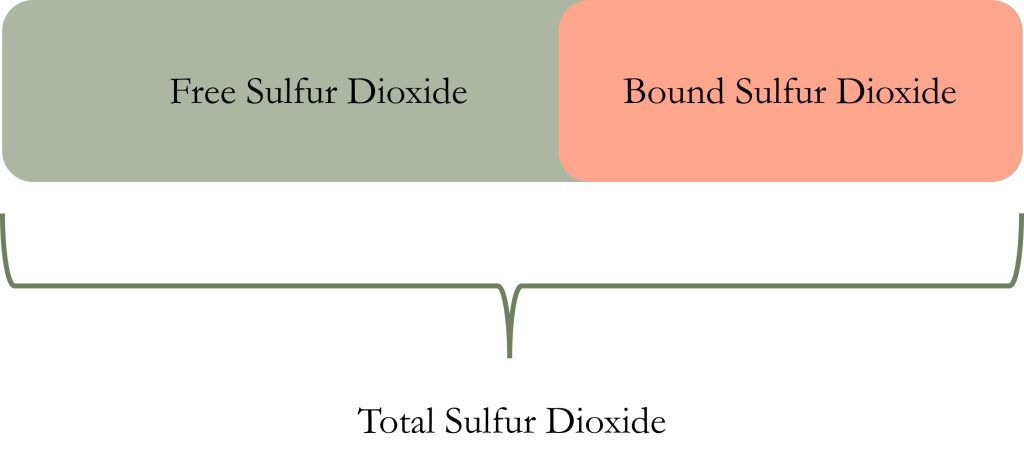
Image by: Denise M. Gardner
Sulfur dioxide exists in both a free (gaseous, volatile) form and bound form in wine. The bound form is just that: sulfur dioxide bound to some other component in the wine (e.g., tannins, sugars, acetaldehyde, anthocyanins) (Waterhouse, Sacks, and Jeffery, 2016). (Author’s note: the combination of both free and bound SO2 provides the total SO2 concentration, which is regulated in terms of total allowance in a wine via the CFR.)
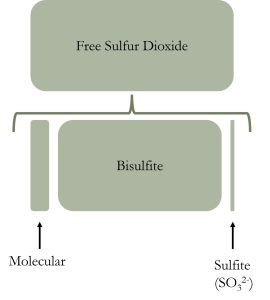
Free sulfur dioxide is further broken down into three components:
- sulfate (which we basically don’t talk about as the concentration is negligible),
- sulfite (that drives the antioxidant power of sulfur dioxide), and
- the molecular form (which determines the microbial strength of sulfur dioxide).
In wine, we aim for one of two molecular concentrations of sulfur dioxide: 0.5 ppm (molecular), which is often recommended for red wines, and 0.80 – 0.85 ppm (molecular), which is often recommended for white, rosé, or sweet wines.
The pH of the wine determines the concentration of free SO2 required to maintain a 0.5 ppm or 0.80 – 0.85 ppm (molecular) concentration of SO2. This is why as the pH increases, the amount of free SO2 required to maintain the same molecular concentration increases.
The 0.80 – 0.85 ppm (molecular) concentration is fully antimicrobial to molds, most yeast, and bacteria that can spoil a wine. Additionally, most of us can smell/taste the SO2 at the 0.80 – 0.85 ppm (molecular) concentration. (Author’s note: More on this winemaking misconception in a future post!)
The 0.5 ppm (molecular) concentration is not fully antimicrobial, but this is the molecular concentration that is targeted for red wines to A) reduce the possibility of bleaching red pigments, mono-anthocyanins, that may be in the wine and B) because the phenolic concentration in red wines also contributes some antimicrobial strength to the wine itself, which is generally lacking in white and rosé wines.
All of these SO2 facts being considered, it is scientifically true that wine is a natural product, filled with microorganisms (molds, yeasts, and bacteria) from the start of harvest, coming in on the fruit, and existing in the wine through bottling operations (and sometimes in the bottle…). Wine is also a transition product, if-you-will, between fresh grape juice and vinegar. That means, wine falls on a spectrum, as a product, between grape juice and vinegar. The microorganisms in the juice, and environmental conditions or processes that surround the juice favor the specifications required to harbor a strong growing environment for winemaking microbes (Saccharomyces yeast) while downplaying the environment that favors vinegar-making microbes (acetic acid bacteria). However, both sets of microbes exist in the juice at harvest and through the winemaking process once the wine becomes wine.
I point out all of this to say: your wine is just one big liquid microorganism party.
While most winemakers conclude, correctly, that some SO2 can bind to naturally occurring components in the wine itself (e.g., tannins, acetaldehyde, sugars), they fail to see how microorganisms and microbial growth can also reduce the efficacy of SO2 adds, too. Many falsely assume SO2 kills microorganisms, when in fact, SO2 is an inhibitory agent when used at proper concentrations. It inhibits microbial growth, but does not significantly kill microorganisms. Thus, the presence of microorganisms themselves, whether visual as a film or not visual at all, can bind to SO2. Furthermore, as the microorganisms grow and metabolize, they may force chemicals into the wine that can also bind to SO2. Thus, this can also reduce the efficacy of SO2, reducing a substantial free SO2 addition, but increasing a substantial amount of bound SO2.
What does this mean from a winemaking perspective?
First, the best way to reduce SO2 binding, and maintaining a low bound SO2 (hence, low total SO2) is to manage wine quality with adequate processing techniques that minimize chemical oxidation. This includes maintaining adequate temperature control while the wine is in storage, minimizing oxygen influx into the wine, and maintaining a free SO2 concentration at appropriate concentrations (the molecular levels based on wine pH).
Second, reduce spoilage. Any time there is spoilage growth, there will be a reduction in SO2 efficacy meaning that the free SO2 increases minimally, but the bound SO2 could increase substantially. How does one reduce spoilage? Manage oxygen, manage wine temperature during tank/barrel storage, reduce oxygen, maintain an adequate free SO2 concentration (molecular concentrations based on wine pH), and make sure there are sound, verifiable sanitation techniques in place in the winery.
Finally, because we now know that chemical processes and microbial growth in wine can reduce the amount of SO2 that may go towards free SO2 during an addition… measure the free SO2 and bound SO2 24 – 48 hours after an SO2 addition. Stop assuming a 25 ppm add brought the free SO2 up to the concentration that was required; find out if the add was substantial enough to compensate for any chemical or microbial processes. (This is the winemaking version of the age old adage, “trust, but verify”.) And, of course, double check there is no stratification in the tank.
Are these the “sexy” topics that we love to talk about with wine? No. But, improving SO2 management is usually one of those things that makes a HUGE turn in wine quality for many. Understanding sulfur dioxide is one of the keystones to winemaking success. Without such knowledge, winemakers are often guessing or over-simplifying a very complex subject that stands between sound winemaking practices and simply getting lucky with wine preservation… sometimes.
Key Take-Aways & Practical Applications
- Always mix the sulfur dioxide into your wine-holding vessels.
- With large tanks, take sulfur dioxide measurements at the top and bottom of the tank to ensure the wine is not experiencing stratification of sulfur dioxide concentrations.
- Measure sulfur dioxide concentrations about 48 hours after an addition. Adjust the SO2 concentrations as necessary.
Resources that Supported this Blog Post:
Harmon, K. 2019. The Effects of SO2 Dosing after Fermentation on Chemistry, Aging, and Sensory Characteristics of Virginia Cabernet Sauvignon. Available at: https://winemakersresearchexchange.com/library/post-fermentation-and-aging/the-effects-of-so2-dosing-after-fermentation-on-chemistry-aging-and-sensory-characteristics-of-virginia-cabernet-sauvignon-2019
Waterhouse, A.L., G.L. Sacks, and D.W. Jeffery. 2016. Understanding Wine Chemistry. ISBN: 978-1-118-62780-8
The views and opinions expressed through dgwinemaking.com are intended for general informational purposes only. Denise Gardner Winemaking does not assume any responsibility or liability for those winery, cidery, or alcohol-producing operations that choose to use any of the information seen here or within dgwinemaking.com.
